Regional Technology and Innovation Hubs
The Problem
Opportunity for National Impact for a Resilient Drug Supply
Access to medicines is a global problem under the current centralized, offshored supply chain, with significant national security risks for the Nation.
- Off-shoring manufacturing efforts have been decades-long in the making in the United States and driven by lower labor & operating costs outside the U.S.
- New investments in U.S. pharmaceutical manufacturing have to compete against existing plants in India and China, that may be outdated, but which have already been fully paid for (including through direct capital support by foreign governments).
- Several hundred drugs are in short supply annually in the U.S., largely due to quality problems associated with manufacturing of the finished dosage form.
- New manufacturing of finished drug product necessitates robust infrastructure for manufacturing, as well as testing, which creates a barrier to reshoring pharma manufacturing.
Solving for this challenge and rebuilding U.S. infrastructure, expertise and workforce requires a diverse, collaborative multi-stakeholder group to drive
Launched following a robust strategic plan funded by the Commonwealth in 2021, we are building on the foundational work of the Alliance for Building Better Medicine and our shared goals to reshore pharma manufacturing and create a resilient drug supply through a globally competitive Advanced Pharmaceutical Manufacturing (APM) Tech Hub.
The Solution
EDA Tech Hub Designation
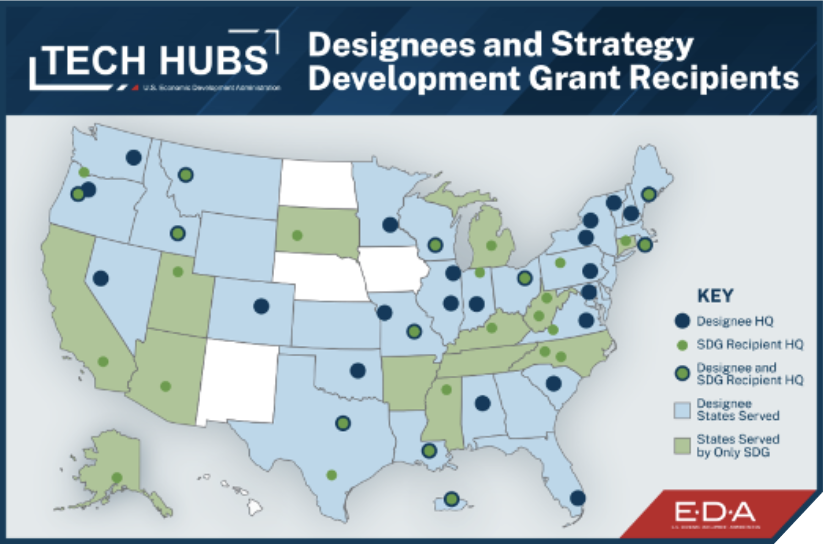
In October 2022, the Biden-Harris administration, through the U.S. Department of Commerce’s Economic Development Administration (EDA), designated the Richmond-Petersburg, VA Region as an APM Technology and Innovation Hub (Tech Hub).
This designation builds on >4 years of co-creation and success — including the win of the EDA’s Build Back Better Regional Challenge (BBBRC) in 2022, where the region is 1/21 BBBRC Winners.
- The APM Hub is 1/31 Designated Tech Hubs from nearly 400 applicants
- Ours is the only Hub addressing innovations that will drive U.S.-based Pharmaceutical manufacturing of lifesaving and life-sustaining medicines.
2024 Overarching APM Tech Hub Objectives
In our Case for Designation, we presented the following three objectives we will realize to achieve global competitiveness in APM in the next decade:
- Accelerate and expand the Consortia’s capacity to deploy and commercialize APIs
- Catalyze additional job growth and investment (supporting up to 5,500 new direct biopharma jobs in the next decade)
- Become a global leader in research and end-to-end manufacturing of medicines
Consortia Members
The Tech Hub effort is led by the Commonwealth Center for Advanced Manufacturing (CCAM) on behalf of the Alliance for Building Better Medicine (Alliance), this diverse consortia includes more than 40 organizations to date.

Additional Support
Component Project Applications
Designation affords the Hub the opportunity to apply for $40-$70M in implementation funding through 3 to 8 integrated component projects, among other Federal support offered.
Through 7 integrated component projects, led by a diverse set of Alliance member organizations, we will drive the next phase of our strategic plan, complementing BBBRC project investments and our pending NSF Engines Type-1 Application.
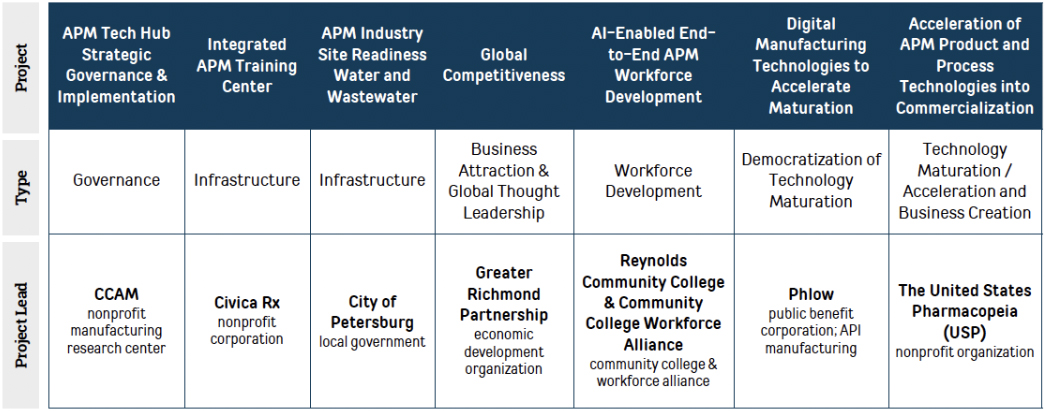
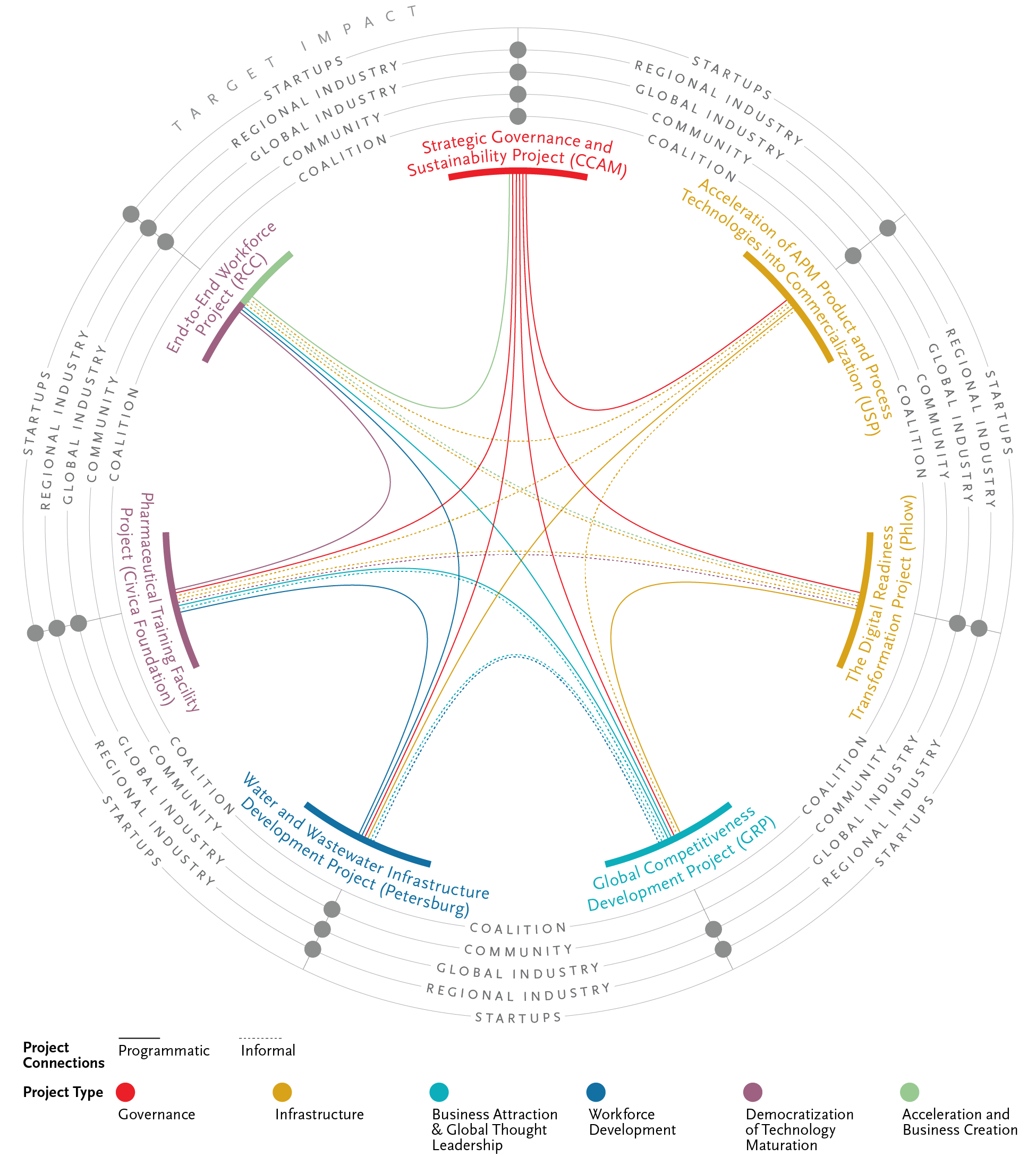
Our APM Tech Hub proposal builds on the developments and learnings over the past five years. We have brought together more APM elements in this Region than anywhere in the world, including $964M in investments. With this strategic EDA Tech Hub investment of these seven thoughtfully designed and interconnected CPs, pictured below, we believe we have the capabilities and assets to sustain and surpass our Region’s acceleration to becoming the global technology hub in APM, with a strong emphasis on the accelerated commercialization work that will drive a true bench to medicine cabinet Tech Hub for the nation.
Resources
Additional Policy Resources
For more information, please refer to the following relevant USP policy articles through their embedded links to the USP Website:
- Identifying and addressing vulnerabilities in the upstream medicines supply chain to build resilience and reduce drug shortages
- Recognizing Challenges and Opportunities to Support Adoption of Advanced Manufacturing Technologies for Medical Products
- Building geographic diversity in the medicines supply chain
- United States Pharmacopeia (USP) Statement for the Record Submitted to the Senate Finance Committee for the Hearing: “Drug Shortages: Examining Supply Challenges, Impacts, and Policy Solutions from a Federal Health Program Perspective” December 5, 2023
